According to the World Health Organization, over one billion out of the world’s seven billion people do not have access to safe drinking water. Water is a fundamental human right. Water chemistry can play a significant role in the sustainability of water resources. Water acquires its chemical compositions and isotopes through reactions with rocks, soils, and sediments (discipline jargon "water-rock interactions") or from anthropogenic discharge. Chemicals in water determine water quality, and chemicals and isotopes are tracers for quantifying the water fluxes moving from one reservoir to another.
Over one billion out of the world’s seven billion people do not have access to safe drinking water. Water is a fundamental human right.
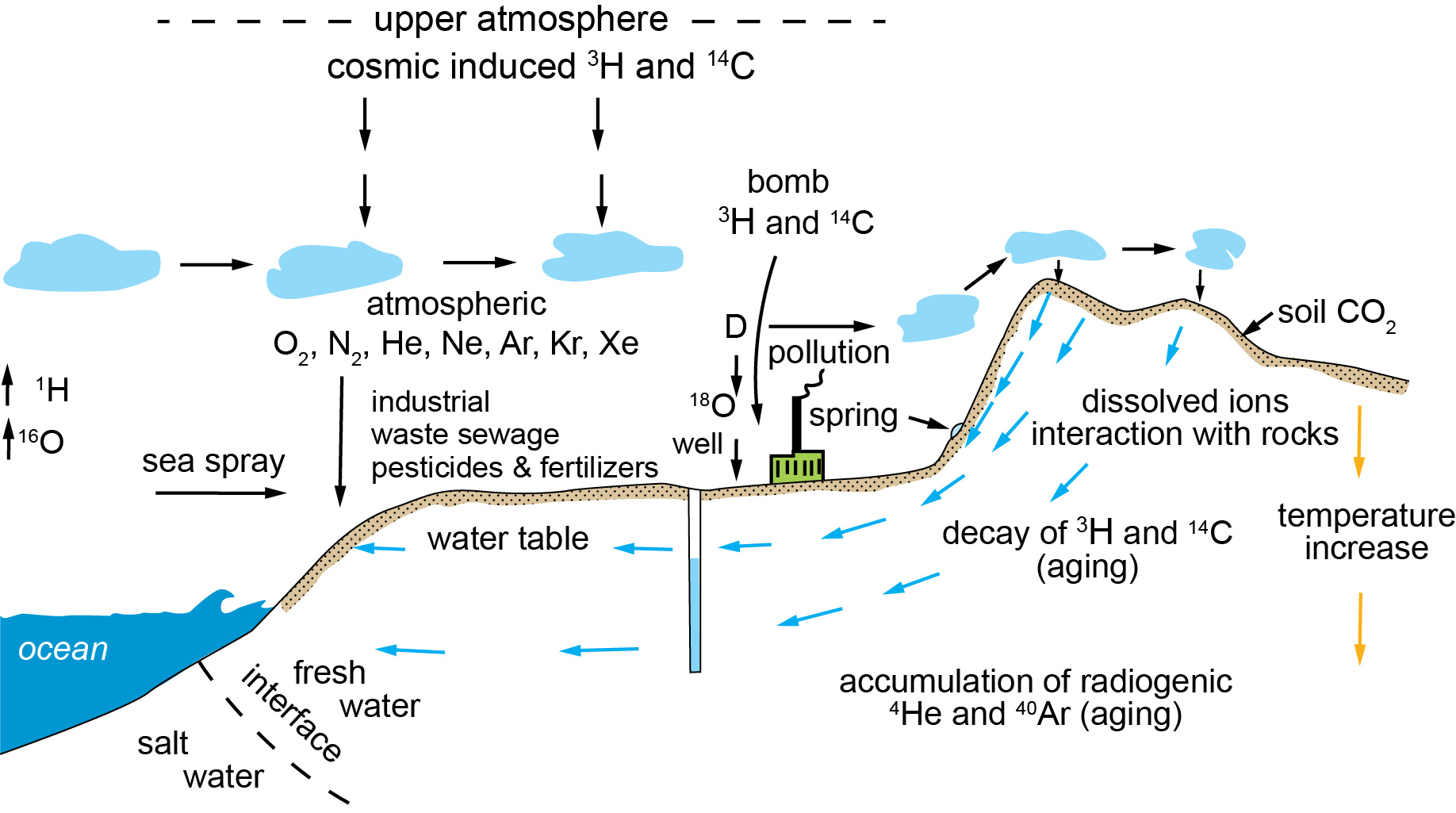
My research characterizes the chemical and isotopic compositions of natural waters and deciphers the chemical processes and mechanisms that determine these characteristics. My past projects and contributions in this area are the innovative and quantitative applications of 14C, 36Cl, 18O, 2H, Cl, and noble gases to studies of hydrological processes, and derivation of in situ reaction rates. The basic scientific questions that my work addresses are how chemical reactions actually proceed in relative accessible geological systems and why in situ reactions proceed at rates that are orders of magnitude slower than those that are measured in laboratories. I have also studied groundwater contamination of arsenic, antimony, and uranium from manufacturing and mining processes. My current interests in this area include continued research on the question of field-lab rate discrepancy and on water quality and water resource management at the watershed scale.


